Metabolisms change chemistry
So, when you breathe in oxygen, that element goes into your lungs and, in those tiny capillaries, it gets exchanged for carbon dioxide (CO2). You breathe out air with more carbon dioxide in it. The oxygen provides electrons to cellular processes that are the electrical current of your body, that runs all your life processes. That is, in short, your metabolism. When you breathe out that CO2, you change the chemistry of the air just outside of your mouth. Here’s a nice, short video that shows how that air becomes more acidic with the addition of that CO2. So that’s the first thing you need to know about my research: organisms, through their metabolisms, change the chemistry of their own, very local environment.
The formation of calcite is super important
The second thing you need to understand is the formation of a very common mineral: calcite or calcium carbonate, CaCO3. It is the main building block of the rock limestone. Corals, clams, and many other organisms secrete it to make their shells or homes. If you drop 10 percent hydrochloric acid on it, it fizzes. Most calcite forms in a chemical reaction with water. If you can bare with me, I hope you’ll find that these chemical equations can really help you understand what’s going on here:
First, just look at the water and carbon dioxide, the top row, of those equations. This basically says that CO2 mixes with water. You know this every time you pop open a soda. The CO2 in the soda comes out of solution and makes the bubbles. You will also notice that most sodas taste a bit acidic. The more CO2 in the water, the more carbonic acid in the water and the more the water can dissolve things. The opposite is also very true: with less CO2, that water becomes less acidic.[1]
Okay, now that second line. If the water has a lot of calcium in it and if there isn’t too much carbonic acid, the water becomes enriched, instead, in a molecule (ion, really) called bicarbonate. This tends to happen when the availability of CO2 is relatively low. You might think of this like the conditions that allow salt to form from evaporating seawater. As the seawater evaporates, the sodium (N) and chloride (Cl) ions get more concentrated and, poof!, salt crystals form and settle to the bottom of the lake/salt flat/test tube. The same thing happens with calcium and bicarbonate: if the water evaporates it changes the concentration of Ca and bicarbonate, triggering the formation of the mineral calcite on the right side of that reaction!
For our purposes, what is really important is CO2. If you change the amount of it in the water, you can make it easier or harder to form the mineral calcite. Okay. That’s all the chemistry.
Microbial mat communities
Microbes are probably the most pervasive, ubiquitous, and, by volume and weight, biggest type of life on Earth. They almost never live alone. They live in slimy coatings just about everywhere. I’m interested in the ones that live in lakes. These groups of microbes live in communities called microbial mats and they are truly teaming with different species of microbes. I sampled a slimy mat in a modern lake in Nevada and we were able to find well over 100 species in it by looking at the genetic (RNA) composition of the slime.
Now, here’s the deal: humans metabolize oxygen to produce CO2. Plants metabolize CO2 to produce oxygen (photosynthesis). There are organisms, mainly microbes, that metabolize sulfur, iron, and other elements to survive. Some like to live in places with a lot of oxygen, called oxic environments. Others don’t like it so much, so they live in anoxic environments. So some organisms are oxidizers (we are oxidizers) and some are reducers (we have bacteria in our gut that are reducers, making our excrement, well, stinky). In a microbial mat you will find microbes that do it all: photosynthesizers, oxidizers, reducers, chemosynthesizers, etc. Like humans and plants, they live off of the waste of the others. This is often considered a symbiotic system.
If you made it this far, congratulations. Here is the big take home point:
Microbial mat communities, through their interacting metabolisms, change the chemistry of the water around them. These changes can lead to the formation of minerals like calcite, through the reaction(s) above.[2]
…and if you form calcite, you can form rocks. Those rocks are limestones and if the microbial communities leave traces of themselves behind, then those rocks are microbialites. That’s what I’m studying over here in Spain.

Who cares?
You might think that this kind of work is really obscure. Why the hell would anyone get paid to go to Spain to study these rocks? It’s a very reasonable question. There are few answers:
1. Humans like me are curious. We want to know how things work. When we see something we are compelled to try to explain it. This is the fundamental basis of science.
2. Microbes are the most common type of life and, until demonstrated otherwise, they are what every other type of life evolved from. So their fossil record can shed light on that evolution and what the environment was like during the earliest times in Earth history.
3. If we’re going to find evidence for life on another planet or celestial object, the heavy odds-on favorite will be microbialites. This is because they are the simplest forms of life, the most common, and the most pervasive. Indeed, the Mars rovers have both been looking for microbialites as they cruise around out there.
4. There are at least two economic reasons for studying microbialites: petroleum exploration and lithium mining. The best crude oil comes from fossil microbes and, as it turns out, there are some important oil reservoirs that have formed in microbialites from ancient lakes like those that I study. If there are volcanic eruptions that happen during the formation of some microbialites, these eruptions can provide lithium to lake deposits. This lithium can get concentrated near zones of microbialite formation. And lithium is, as you know, vital for the construction of batteries.
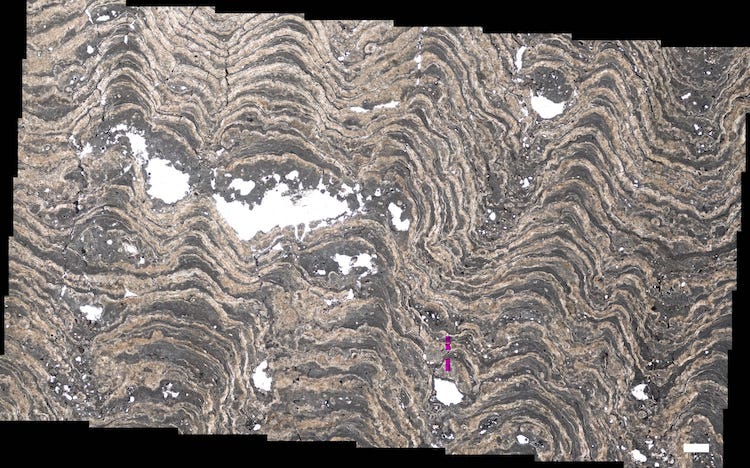
Why Spain?
I work on a fairly specific type of microbialite deposit: those that form in lakes. I am also very interested in how these rocks vary over distance, not time. So I have to find rock outcrops where there is high confidence that we know that we’re looking at basically the same lake. I can’t just go through a bunch of sedimentary layers, because each layer represents different time periods. I really want one layer that I can trace as far as possible. Like that Martin the Martian Microbialite above. It came from a single layer that I can trace over about 5 miles. This helps me understand the normal variability in microbialite types that one might find in a single lake. So to research these, I need to find very very specific outcrops that satisfy my needs.
One place in the world that does this is in northern Spain. In particular, there are several places near Madrid and Zaragoza that, hopefully, will work for a study. So next week I head out into the field with Concha Arenas-Abad, a professor at the University of Zaragoza. She has agreed to show me around some outcrops from the Ebro Basin and I hope to collaborate with her on a research paper, based on the work that I do here. In a few more weeks I plan on going out in the field with Esther Sanz-Montero to check out localities in the Madrid and Duero Basins.
[1] An interesting side comment: as humans put more carbon dioxide into the air by burning fossil fuels, they make the atmosphere more acidic, which makes the ocean surface more acide, which causes corals to die: coral bleaching. Warmer water makes all of these reactions go faster.
[2] This is different from what corals and clams do, where their cells actively play a role in the formation of calcite. For them, it happens at the genetic level and is like a cellular machine. For microbial mat communities it is more passive: the community changes the chemistry of the surrounding water and it may or may not trigger the formation of minerals like calcite.





